|
|
Proteomic Markers Might Predict Clear Cell Renal Cell Carcinoma Survival - Cancer Therapy Advisor |
February 28, 2015
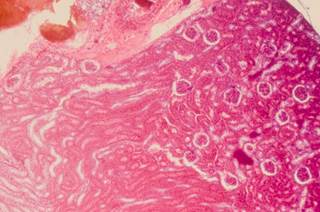
Protein expression markers predict disease-specific survival in clear cell renal cell carcinoma.
ORLANDO—Protein expression markers predict disease-specific survival (DSS) in clear cell renal cell carcinoma (ccRCC), according to research (Abstract 406) presented during the 2015 Genitourinary Cancers Symposium.1
“We have identified and validated proteomic signatures which cluster ccRCC patients into five prognostic groups,” reported lead study author Samuel D. Kaffenberger, MD, of the Memorial Sloan Kettering Cancer Center in New York, NY. “Furthermore, two distinct mTOR-activated clusters—one with high receptor tyrosine kinase (RTK) activity and one with increased mTOR pathway genomic alterations—were revealed, which may have prognostic and therapeutic implications.”
The study authors ”sought to leverage reverse phase protein array (RPPA) data from The Cancer Genome Atlas (TCGA)” with an independently developed proteomic platform to identify druggable targets and pathways associated with prognosis in ccRCC, Dr. Kaffenberger said.
They conducted pathway analysis for differentially expressed proteins and assessed associations with clinicogenomic factors and DSS.
“RPPA clustering of 324 patients from the ccRCC TCGA [cohort] revealed five robust clusters characterized by alterations in specific pathways and divergent prognoses,” Dr. Kaffenberger explained.
Both clusters 1 and 2 showed evidence of downstream upregulation of mTOR.
Cluster 1 was characterized by poor DSS, decreased expression of RTK and upregulation of the mTOR pathway, and was associated with mTOR pathway genomic alterations, sarcomatoid histology, and the ccB prognostic mRNA signature (all Ps <0.001).
Cluster 2 was characterized by increased expression of RTKs “and interestingly, had upregulation of the mTOR pathway with excellent DSS,” Dr. Kaffenberger noted. Whereas cluster 1 was associated with worse survival, cluster 2 was associated with superior DSS. After accounting for stage and grade in multivariate analysis, cluster designations remained independently associated with DSS (hazard ratio=0.23 for cluster 2; 95% CI 0.08-0.68; P=0.008).”
“Cluster 1 was associated with universal downregulation of the RTK pathway,” Dr. Kaffenberger noted. “Conversely, cluster 2, which was also associated with upregulation of the mTOR pathway, was associated with universal upregulation of the RTK pathways.”
The study authors subsequently performed an external validation analysis with a separate cohort of 189 patients, using a different proteomics platform—a panel of phosphoproteins (pHER1, pHER2, pHER3, pSHC, pMEK, pAKT) that were highly discriminant between clusters 1 and 2, Dr. Kaffenberger reported.
RELATED: Adjuvant Sorafenib, Sunitinib for Advance Renal Cell Carcinoma 'Should Not Be Pursued'
In the external validation cohort, patients with mTOR pathway activation were segregated into two groups: those with coincident high-RTK activation (83 patients) and those with low RTK activation (13 patients). A univariate comparison of DSS in these high- versus low-RTK score groups found a significant difference: DSS hazard ratio=0.19 (95% CI: 0.05-0.082; P=0.03).
“We found that we were able to accurately stratify patients with clear cell RCC based purely upon protein expression, and furthermore, we found that this stratification was independently prognostic,” Dr. Kaffenberger concluded. “We validated this data with a separate patient cohort and a separate proteomics plastform.”
Following the external validation analysis, RPPA was adapted to predict responses to mTOR inhibitor (mTORi) therapy, using a novel RPPA antibody panel. Preliminary results suggest the novel RPPA panel can predict response to mTOR inhibition, and the researchers are now pursuing study of a larger cohort of mTORi responders.
Reference
Kaffenberger SD, Ciriello G, Winer AG. Proteomic stratification of clear cell renal cell carcinoma utilizing The Cancer Genome Atlas (TCGA) with external validation. 2015 Genitourinary Cancers Symposium. Abstract 406.
|
|
|
Adjuvant Sorafenib, Sunitinib for Advance Renal Cell Carcinoma 'Should Not Be ... - Cancer Therapy Advisor |
February 28, 2015

Adjuvant sorafenib and sunitinib do not improve survival for patients with locally advanced renal cell carcinoma.
ORLANDO—Adjuvant sorafenib and sunitinib do not improve survival for patients with locally advanced renal cell carcinoma (RCC), and were associated with frequent dose reductions and treatment discontinuations, according to initial data from the ASSURE clinical study presented during the 2015 Genitourinary Cancers Symposium.1
“Findings from this study suggest that patients with locally advanced kidney cancer should not be treated with either adjuvant sorafenib or sunitinib,” reported study author Naomi B. Haas, MD, of the Abramson Cancer Center of the University of Pennsylvania in Philadelphia, PA. “Median time to disease recurrence did not differ between those who received sorafenib or sunitinib after surgery (median 5.8 years each) and those treated with placebo (median 6 years).”
“Dose titration reduced the treatment discontinuation rate; this finding may have relevance in other settings,” she noted. “This was the first and largest trial reporting on the efficacy of VEGF inhibitors as adjuvant therapy for patients with locally advanced kidney cancer who are at high risk of recurrence.”
Because locally advanced RCC is not always cured by surgery, the study authors investigated the oral agents, which are “widely effective” in the metastatic setting, as potential adjuvant therapies.
A total of 1,943 patients with completely resected RCC (pT1b high grade to pT4 any grade N any) were stratified by risk category (intermediate high or very high), clear/non-clear histology, ECOG PS, and resection approach, and were randomly assigned equally to sunitinib daily for 4- or 6-week cycle, sorafenib daily, or placebo, Dr. Haas reported.
However, after 1,322 patients had been enrolled, “the starting dose was reduced and then individually titrated to mitigate the effect of patient discontinuation from treatment intolerance,” Dr. Haas noted.
Participants received assigned treatments for up to 1 year. Disease-free survival (DFS) was the primary study endpoint.
RELATED: Renal Cell Carcinoma Risk Inversely Linked with Lycopene
When the study had accumulated two-thirds of the planned data, an interim analysis was conducted. Efficacy and futility boundaries had not been crossed but the study's safety monitoring committee recommended release of results.
“There were no significant differences in DFS or overall survival between either of the experimental arms and placebo,” Dr. Haas said. A subgroup analysis of DFS for clear cell RCC similarly found no significant differences among patients administered placebo and those administered adjuvant sunitinib or sorafenib.
“All three study arms did better than expected” in terms of 5-year overall survival, Dr. Hass noted—but there were no significant differences in OS between the experimental and placebo study arms (5-year OS: sunitinib: 76.9% [95% CI: 72.9-81.2%]; sorafenib: 80.7% [95% CI: 77.0-84.6%]; placebo: 78.7% [95% CI: 74.8-82.8%]).
“There is a suggestion that women performed worse on sorafenib than placebo,” Dr. Hass noted.
Frequent grade 3 or higher adverse events included hypertension (16% sunitinib/16% sorafenib/4% placebo), hand??-foot reaction (15%/33%/1%), rash (2%/15%/<1%), and fatigue (17%/7%/3%), Dr. Haas said.
Reference
- Manola J, Uzzo RG, Flaherty K, et al. Initial results from ASSURE (E2805): Adjuvant sorafenib or sunitinib for unfavorable renal carcinoma, an ECOG-ACRIN-led, NCTN phase III trial. 2015 Genitourinary Cancers Symposium. Abstract 403.
|
|
All day Mod music festival in Redcar raises funds for the Pete Quaife Foundation - Gazette Live |
|
 VIEW GALLERY VIEW GALLERY
The brother of founding member of The Kinks Pete Quaife rocked into town for an all day Mod music festival.
Held at Redcar’s Coatham Memorial Hall, the 12-hour festival started at 11am today and included 11 acts and three DJs.
Bands scheduled to perform at the All Day and All of the Night festival include The Universal, Jacques and the Giants, The Last Fakers, King Mojo, Heavy Mod, The Transmitters, The Clashed, Alistair Sheerin, Acoustic Weller, The Whodlums and The Lemontops.
And keeping the music going in between were DJs Alan May - known as Glory Boy - and Mick the Mod playing the best in ska, mod and Northern Soul.
About 250 people were expected to be at the music event which was held to raise funds for the Pete Quaife Foundation, which gives children on dialysis treatment entertainment while undergoing their four hours of treatment three times weekly - providing equipment such as portable DVD players and Kindles that can be sterilised.
David Quaife, Pete’s brother, said he was thrilled to be at the music event and was enjoying a weekend in Teesside.
“The money raised will go towards children in this area. We’re going to organise an event in June or July, take them on a trip out in a bus wherever they want to go and I’ve even twisted the arm of the dietician at Newcastle so they can have a picnic too.
“The guys who organised this event are the tops.”
DJ Alan May said: “Once you’re on the Mod scene you’re in it for life.”
Mark Simpson, a member of event organisers The Collective Few, said: “This is the first all day event we’ve held and we’re hoping to hold more in the future.
“We’re hoping to raise £4,500 for the foundation.
“Tesco has donated lots of raffle prizes and Redcar Development Trust have helped us so much too.”
|
|
State Health Officer Announces Certificate of Need Decisions for Projects in ... - WDAM-TV |
|
JACKSON, MS (WDAM) -
This is a news release from the Mississippi department of Health
The Mississippi State Department of Health (MSDH) announces the issuance of a Certificate of Need (CON) for the following projects in Vicksburg and Louisville.
Vicksburg Healthcare, LLC
Vicksburg, Mississippi
Cost Overrun to CON #R-0871 (CON Review #HG-MOB-0913-012), Construction of River Region Medical Office Building
Vicksburg Healthcare, LLC received Certificate of Need (CON) authority for the cost overrun to CON #R-0871 (CON Review #HG-MOB-0913-012) for the construction of a medical office building for River Region Health Systems. The applicant states that based on soil composition, additional work was needed to prepare the site for construction and other costs were incurred.
The original capital expenditure was $13,245,099. The additional capital expenditure is $3,723,912. The total capital expenditure for this project is $16,969,011.
RCG Mississippi, Inc. d/b/a RCG Louisville
Louisville, Mississippi
Repair of ESRD Facility Due to Natural Disaster and Expansion
RCG Mississippi, Inc., d/b/a RCG Louisville received Certificate of Need (CON) authority to repair its 5,300 square foot Winston County facility due to extensive damage caused by an EF4 tornado in April 2014. After repairs are completed, the project will also include the addition of 1,565 square feet of space to replace the remaining four stations and accommodate four new ESRD (end stage renal disease) stations, providing a total of 21 ESRD stations.
The total capital expenditure for this project is $1,483,261.
Mississippi's Certificate of Need process is a fundamental component of the state's health planning and health regulatory activities. In managing the Certificate of Need process, the Department seeks to improve the health of Mississippi residents; to increase accessibility, acceptability, continuity and quality of health services; to prevent unnecessary duplication of health resources; and to provide some cost containment.
The MSDH has administered the Certificate of Need program since July 1986. Since then, more than 1,400 Certificate of Need applications have been reviewed, representing total capital expenditures of approximately $5 billion. The next Certificate of Need monthly meeting will be March 26, 2015, at the MSDH offices in Jackson.
The department's staff analysis for each Certificate of Need application is published online atwww.HealthyMS.com.
Copyright 2015 WDAM. All rights reserved.
|
|
Making Bali Accessible to Dialysis Patients - Bali Discovery |
|
 Making Bali Accessible to Dialysis Patients Making Bali Accessible to Dialysis Patients
Bali Discovery
People suffering from chronic kidney failure benefit from life-saving dialysis treatment in which routine treatment allows people undergoing such care to enjoy the many joys of normal life – including travel. Those needing dialysis can now travel with ...
|
|
|
|
|
|
<< Start < Prev 211 212 213 214 215 216 217 218 219 220 Next > End >>
|
|
Page 217 of 2630 |
 Log in to explore the world's most comprehensive database of dialysis centres for free!
Log in to explore the world's most comprehensive database of dialysis centres for free!  Professional dialysis recruitment
Professional dialysis recruitment



